Phyto-pharmaceuticals – Herbal Medicinal Products
Herbal medicinal products are pharmaceutical products of plant origin, also known as phyto-pharmaceuticals. They exclusively contain one or more herbal substances or herbal preparations as active ingredients or combinations of them (1).
June 14, 2017
In contrast to synthetic drugs containing chemically active substances, they consist of a plethora of natural compounds that activate or modulate various target systems in an organism. Herbal medicinal products can be variable in their composition. Therefore, to obtain consistent efficacy and safety, standardised medicinal plant extracts are being used. Evidence-based phytotherapy makes use of scientifically verified efficacy and safety by randomized controlled clinical trials. In general, herbal medicinal products are better tolerated and provide a superior benefit-risk ratio to synthetic drugs. Therefore, evidence-based phyto-pharmaceuticals increasingly mentioned in clinical practice guidelines are first-line therapy in various diseases and indications.
The term phyto-pharmacon / phyto-pharmaceutical is derived from the Greek designations phytón for plant and phármakon for medicine. Basically, these are herbal remedies which are prepared from herbal substances like dried plant parts such as leaves, blossom, herb, bark, or the roots traditionally already known to cure undesired conditions or illnesses. Today, most herbal preparations are extracts from herbal substances in optimized form with known extraction solvent, drug-extract ratios and processing steps finalized as pharmacologically active dry extract.
Highest quality (GMP), clinically confirmed efficacy and best tolerability and safety of standardized or quantified medicinal plant extracts are key measures for a successful phytotherapy approach. Other than food supplements or botanicals phyto-pharmaceuticals have to follow comparable regulatory guidelines as conventional drugs. Therefore, a high level of scientific evidence supports their efficacy and safety. For example, the St. John’s wort extract Ze 117 shows comparable efficacy as the synthetic antidepressant drugs imipramine or fluoxetine (SSRI) in the treatment of depressive disorders (2, 3). St. John’s wort (Hypericum perforatum L.) extracts have been attested with the well-established use status by the EMA (4) and have reached Level A evidence (5) of efficacy as they confirmed to be superior to placebo in patients with major depression. Further, they are similarly effective as standard antidepressants and have fewer side effects as concluded in a Cochrane meta-analysis (6).
Importantly, evidence-based phyto-pharmaceuticals should be clearly separated from alternative or complementary therapeutic approaches such as homeopathy.
Phyto-pharmaceuticals are complex mixtures
Unlike synthetic drugs, which are single active chemical substances, phyto-pharmaceuticals contain complex mixtures of hundreds of natural components. Among them, several isolated lead substances are well-known to be pharmacologically active while others are still under scientific investigation. The efficacy of herbal medicinal products results from complex interactions the active components with molecular target structures, such as receptors, enzymes, and transport systems. Herbal medicine was also the origin of conventional academic medicine. Even today, about 70% of medicines contain active ingredients that were originally derived from natural substances. Many classical active components, such as the pain reliever morphine, the cardiac glycoside digoxin or the anticholinergic agent atropine originate from plants. Today, however, such isolated substances are no longer considered as phyto-pharmaceuticals as they are either produced by chemical synthesis or are isolated and purified to be used as single substances.
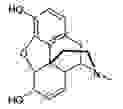
Morphine
The extract as active principle
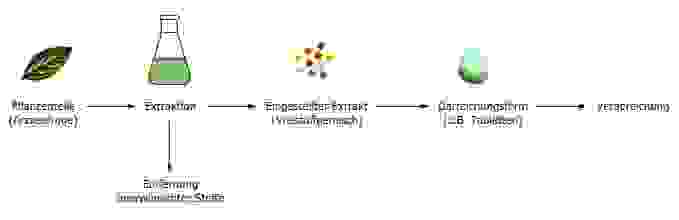
Phyto-pharmaceuticals are natural products and like other natural products, such as coffee, wine or cocoa, their quality depends on many factors starting from the raw material. For example the plant variety, cultivation, climate, harvest time, drying process and further processing steps can all affect the quality of the final preparation. It is therefore obvious that active ingredients in two different teas made from the same plant type can be present in very different concentrations. Similarly, no coffee tastes the same as another, although they are all prepared from coffee beans. For this reason, increasing numbers of extracts are being manufactured, which are standardised or quantified with regard to important components. Such extracts therefore always contain defined amounts of these active ingredients. Likewise undesired substances; those that cause side-effects, can be removed. Extracts from different manufacturers can thus only be compared with each other to a limited extent. Various different dosage forms, such as film-coated tablets, drops, or ointments, can be manufactured from the extracts.
Phyto-pharmaceuticals are not homeopathic products
Phyto-pharmaceuticals contain pharmacologically active ingredients that interact with protein structures in the human body, the so-called drug targets. They are, therefore, substantially different to homoeopathic remedies, which are highly diluted so that little or nothing of the initial active ingredient is actually left. In contrast to phytotherapy, homeopathy has no solid scientific evidence and is considered an alternative, complementary therapy approach. The concept of homeopathic products is fundamentally contrary to that of modern medicinal products.
Clinically proven phytotherapy
The highest demands are made on clinically proven phyto-pharmaceuticals. Their effect and safety have to be verified in randomized, double-blind, (placebo)-controlled clinical trials. They are developed and scientifically evaluated in the same way as conventional medicinal products. This is very different to traditional phyto-pharmaceuticals, where the use is primarily based on experience, for example the administration of tannin-containing black teas for diarrhoea.
Typical examples of clinically proven phyto-pharmaceuticals are:
John’s wort for the treatment of depressive moods (2-4, 6)
Butterbur for the treatment of hay fever (7)
Gingko for the treatment of declining mental performance (8)
Black cohosh for the treatment of menopausal symptoms (9)
Hawthorn for the treatment of cardiac symptoms (10)
Valerian and hops for the treatment of sleep disorders (11)
Good Tolerance
In principle, phyto-pharmaceuticals show high tolerability and safety but can bear the same risks as other medicinal products. They can have contraindications, there is a risk of undesired side-effects and drug interactions are possible. However, in general phyto-pharmaceuticals are well tolerated and often have a lower risk potential than synthetic chemical medications. Phyto-pharmaceuticals have a broad therapeutic margin and thus work well for simple and chronic symptoms. Due to their high tolerability and rather low interaction potential, they are well suited for elder patients with multiple medications.
References:
- EMA HMPC. EMA/HMPC/402684/2013. 2014.
- Schrader E. Int Clin Psychopharmacol. 2000;15(2):61-8.
- Woelk H. BMJ. 2000;321(7260):536-9.
- EMA HMPC. Community herbal monograph on Hypericum perforatum L.,herba (well-established medicinal use). EMA/HMPC/101304/2008. 2009.
- Kligler B. et al. American family physician. 2016;94(5):369-74.
- Linde K. et al. Cochrane Database Syst Rev. 2008(4):CD000448.
- Schapowal A. BMJ. 2002;324(7330):144-6.
- EMA HMPC. Community herbal monograph on Ginkgo biloba L., folium. EMA/HMPC/321097/2012. 2015.
- Schellenberg R.et al. Evid Based Complement Alternat Med. 2012;2012:260301.
- EMA HMPC. European Union herbal monograph on Crataegus spp., folium cum flore, EMA/HMPC/159075/2014. 2016.
- Koetter U. et al. Phytother Res. 2007;21(9):847-51.
Topics
Filter blog posts by topic by clicking on the tags.